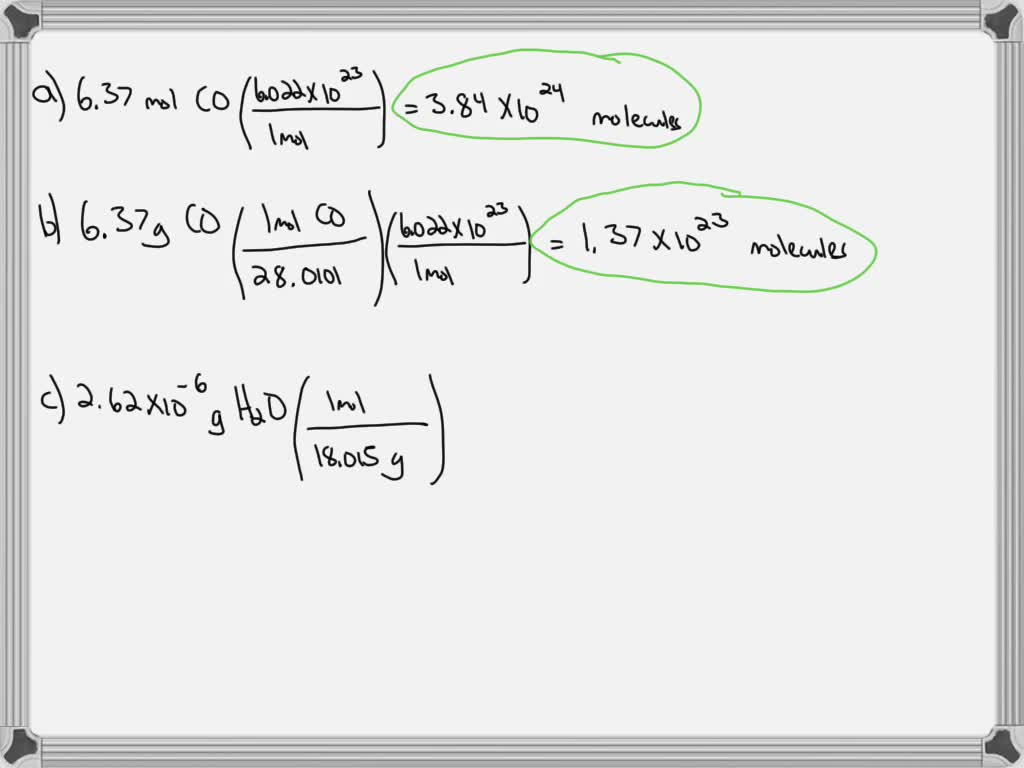
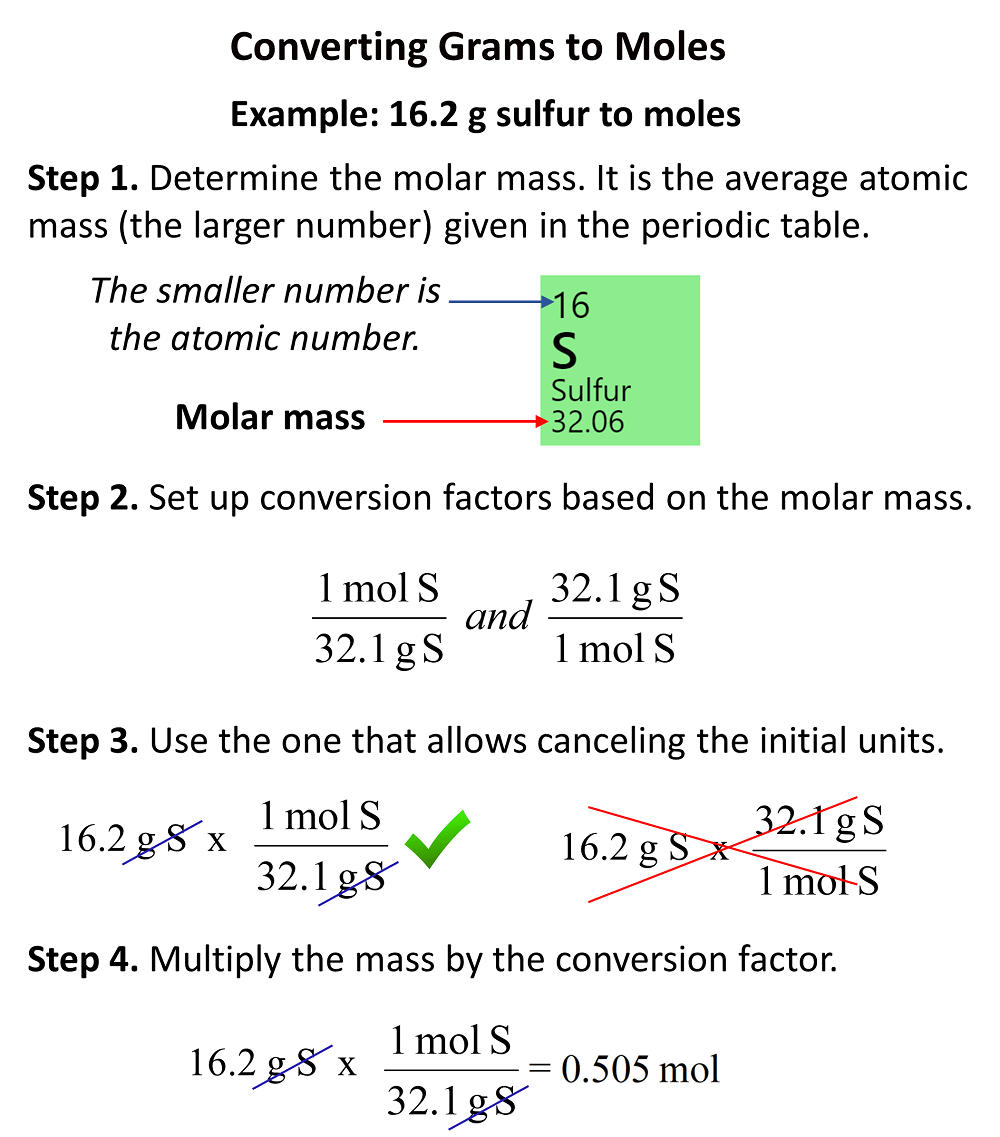
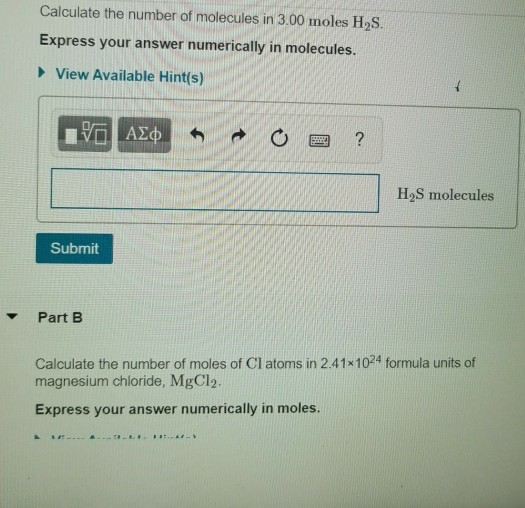
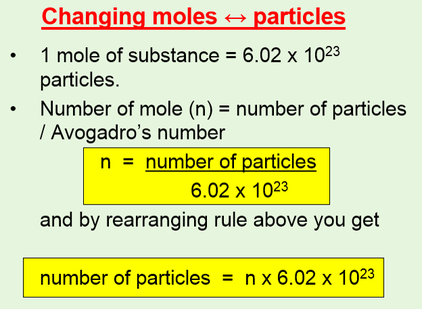
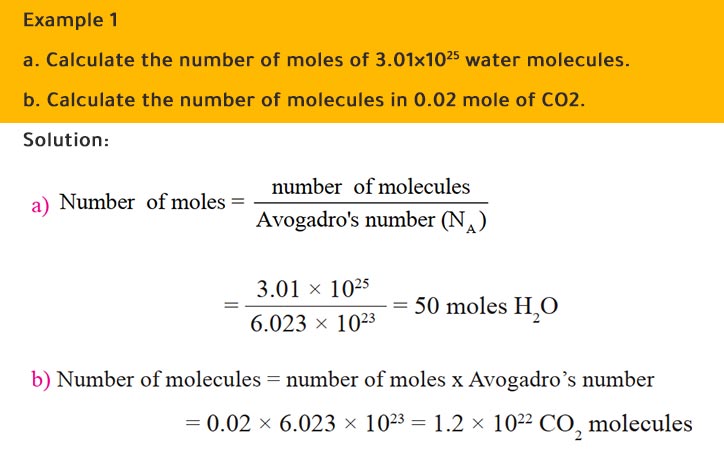
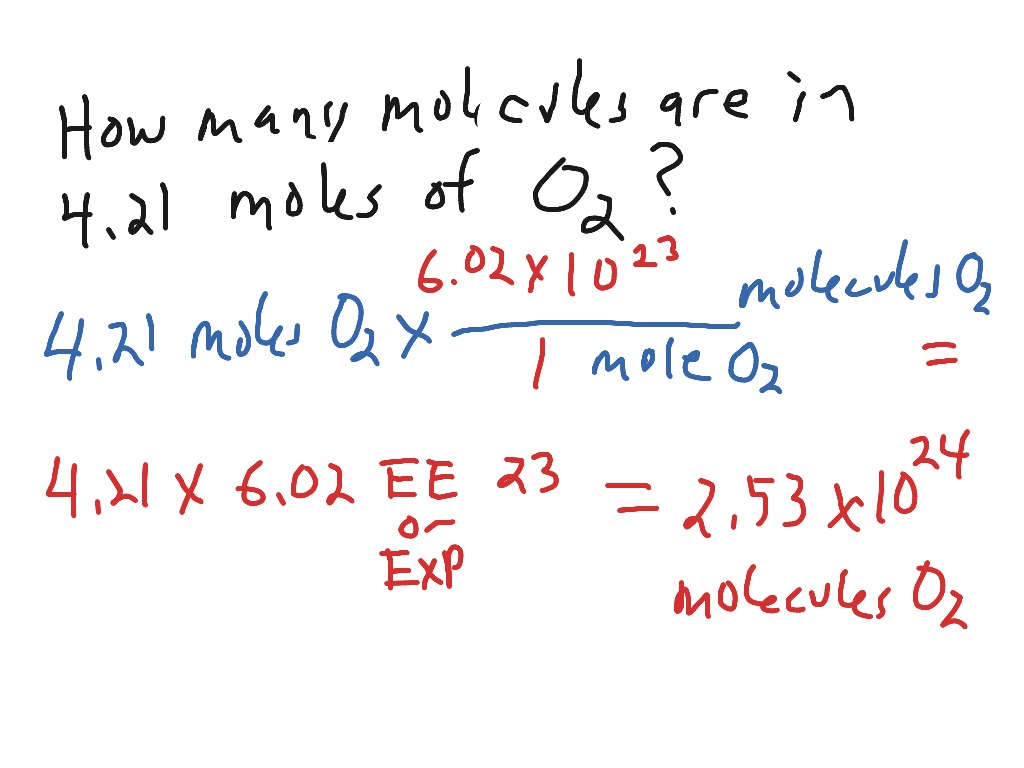SOLVED:Calculate the number of molecules present in each of the following samples. a. 6.37 mol of carbon monoxide b. 6.37 g of carbon monoxide c. 2.62 ×10^-6 of water d. 2.62 ×10^-6

Calculate the number of molecules in 36gm of H2o - Chemistry - Atoms and Molecules - 14431885 | Meritnation.com
Calculate the number of molecules in 2 xx 10^(-6)m^(3) of a perfect gas at 27^(@) C and at a pressure of 0.01 mm of mercury. Mean KE of a molecules at 27^(@)
a) Calculate the mass of 0.5 mole of oxygen atoms. (b) Calculate the number of molecules of glucose present in its 90 grams (molecular mass of glucose is 180 u) (c) Calculate
Calculate the number of particles in each of the following: (a) 48 g of Mg (b) 8 g of O2 (c) 0.1 mole of carbon (Atomic mass Mg = 24 u, O =
How to calculate the number of molecules of an element in a compound if I only know the total mass - Quora

How to calculate Z (the number of molecules in a unit cell) for calculating theoretical density? | ResearchGate